Skeletal muscle cells

Biorelevant culture of muscle cells on Biolaminin substrates
Laminin 211, 221 and 521 form an important part of the adult skeletal muscle microenvironment
Laminin proteins are key components of the basal lamina surrounding muscle stem cells and adult cells (Sanes, 2003 and Yin, 2013). Laminins are not only structural proteins of the basal lamina but are also signaling molecules that are important for the adhesion, localization, and function of muscle cells in their niche. Laminin-211, -221, -511 and -521 are the main laminin isoforms expressed in the basal lamina of striated muscles. Laminin 421 and laminin 111 are also expressed in small amounts, preferably at junctional regions (Rogers & Nishimune, 2016). Laminin 211 and laminin 221 are the most abundant laminin isoform in the adult skeletal muscle basement membrane with integrin α7β1 as the main receptor (Holmberg & Durbeej, 2013) and an expression deficiency in the alpha-2 laminins results in muscular dystrophy, accompanied by a dilated cardiomyopathy (Oliviéro, 2000). Laminin 211 is located within the basement membrane that surrounds the sarcolemma, whereas laminin 221 predominates in the neuromuscular and myotendinous junctions.
Laminin is crucial for normal muscle function, evident from naturally occurring mutations in laminin genes. Mutations in the gene encoding laminin a2 chain are the most common cause of congenital muscular dystrophy type 1A (Gawlik & Durbeej, 2011). In a publication by Fontes-Oliveira et al. (2017), the authors show that the absence of laminin α2 chain leads to downregulated PGC1α expression, which impairs mitochondrial biogenesis, causing a reduction of mitochondrial content that finally leads to a bioenergetic inefficiency in myoblasts and myotubes from MDC1A patients. By culturing MDC1A myotubes on recombinant Biolaminin 211, the mitochondrial function is restored. Basal respiration, maximum respiration, and ATP production, as well as basal mitochondrial respiration and maximal mitochondrial respiration capacity, are normalized to control levels in the presence of laminin 211.
Due to their vital role for muscle tissue structure, maintenance, and function, laminin serve as an important tool in protocols aiming to improve cell therapy for muscular deceases. Moreover, the intramuscular injection of laminin has shown to improve muscle regeneration and the efficiency of myoblast transplantation (Riederer, 2015).
Biolaminin 521 and 221 are superior substrates for both short-term and long-term myogenic cell expansion and differentiation
During myogenesis, laminin 521 and laminin 511 are the main isoforms surrounding myogenic progenitors (Gawlik & Durbeej, 2011). Indeed, Biolaminin 521 cell culture matrix was shown to maintain the differentiation potential of mouse and human satellite cell-derived myoblasts, even during long-term culture expansion (Penton, 2016). Biolaminin 521 supports increased proliferation during expansion and superior differentiation with myotube hypertrophy, larger myotubes and higher amounts of nuclei per myotube. Moreover, Penton et al. show that Biolaminin 521 supports more consistent and reliable differentiation over long-term culture and is the only substrate facilitating high-level fusion following long-term culture. Biolaminin 521 supports increased differentiation potential without altering the traditional Pax7/MyoD paradigm.
Researchers at Stanford, who developed artificial muscle fibers (AMFs) that mimic the native myofiber of the MuSC niche, show that Biolaminin 211 maintains muscle stem cells (MuSCs) in a potent, quiescent state in vitro. AMFs coated with recombinant Biolaminin 211 showed prolonged quiescence in vitro and enhanced potency in vivo (Quarta, 2016).
An improved method for culturing myotubes on laminins for the robust clustering of postsynaptic machinery
Motor neurons from specialized synapses with skeletal muscle fibers called neuromuscular junctions (NMJs). The function of this type of synapse is to transmit signals from the central nervous system to muscles and thus stimulate their contraction. Muscle cells form a thick ECM around the fiber that contains various laminins, collagens, fibronectin, and other glycoproteins. The basal lamina (BL) at the synaptic cleft has a specific molecular composition that contains laminin α4, α5, and β2 isoforms that are mostly absent in extrasynaptic regions of muscle fibers. These ECM components are crucial for the proper development of NMJs and it has been shown that the laminin-dystroglycan interaction is crucial for regulating NMJ developmental remodeling.
Little is known about the mechanisms of postsynaptic machinery remodeling in vivo. Mouse laminin-111 has been routinely used to induce AChR clustering and is the only in vitro system where the AChR clusters undergo developmental remodeling that resembles mature NMJs, providing the model to study the underlying mechanisms. In a publication in Scientific Reports (Pęziński, 2020) the authors present an improved protocol for culturing C2C12 muscle cells that reproducibly promote the formation of complex AChR clusters. The authors tested several laminin isoforms and other matrices, and found that laminin-121 and laminin-221 supported the most developed clusters of postsynaptic machinery in C2C12 myotubes. In addition, laminin-421 and laminin-511 promoted the formation of the most podosome-containing AChR clusters in human primary myotubes. The method may facilitate the identification of novel synaptic regulators. The high reproducibility of culturing and robust formation of AChR clusters with this methods are great features for establishing high-throughput screening. The protocol is also useful for obtaining and freezing a large number of cell stocks and utilizing cells for experimentation with a constant and low passage number, which significantly increases experimental reproducibility. The method can be implemented in different formats, such as permanox slides, glass surfaces as well as multi-well culturing dishes.
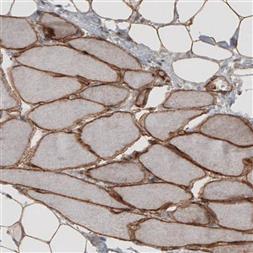
AMAb91096 (Atlas Antibodies).
What our customers say
“Laminin-521 shifts away from the paradigm that muscle cells lose their ability to differentiate after culturing. Muscle differentiation on laminin-521 is nothing short of spectacular, yielding increased numbers of nuclei per myotube and improved myotube organization.”

Dr. Christopher Penton
Icagen, Inc., Tucson, Arizona, USA
Succeed with your application
-
An improved method for culturing myotubes on laminins for the robust clustering of postsynaptic machinery
Pęziński M., DaszczukP., Shankar Pradhan B., Lochmüller H. Prószyński T.J. Scientific Reports, 2020
Read more -
Laminin 521 maintains differentiation potential of mouse and human satellite cell-derived myoblasts during long-term culture expansion
Penton C.M., Badarinarayana V., Prisco J., Powers E., Pincus M., Allen R.E., August P.R. Skeletal Muscle, 2016
Read more -
Instructions 001: Coating with Biolaminin substrates
Protocol and concentration calculations for coating cultureware with Biolaminin
Open pdf
Biolaminin Key Advantages
Myotube culture on Biolaminin 521 gives large myotubes and high amounts of nuclei per myotube. Human myotubes cultured on a combination of Biolaminin-121 and -221 have shown the most developed clusters of postsynaptic machinery.
Specific laminin isoforms are present in different tissue microenvironments and are essential for cell survival, proliferation, and differentiation. Biolaminin products allow you to imitate the natural cell-matrix interactions in vitro.
All our matrices are chemically defined and animal origin-free, which makes them ideal substrates for each level of the scientific process – from basic research to clinical applications.
Our products have consistent composition and quality. This enables minimized variability between experiments and uniform pluripotency gene expression profiles between different cell lines.
Numerous scientists have found our products and finally succeeded in their specific stem cell application. The power of full-length laminins incorporated into various cell systems is well documented in scientific articles and clinical trials.
Recommended products

Biolaminin 121 LN (LN121)
Human recombinant laminin 121
Biolaminin 121 can be used as a general attachment protein for most cell types in vitro – in particular for hepatic and neural differentiation and to enhance neurite outgrowth.View product
Biolaminin 211 LN (LN211)
Human recombinant laminin 211
Biolaminin 211 supports the growth, survival, and differentiation of a wide range of tissue-specific cell types, including motor neurons, cardiac cells, and skeletal muscle cells.View product
Biolaminin 221 LN (LN221)
Human recombinant laminin 221
Biolaminin 221 supports the growth, survival, and differentiation of a wide range of tissue-specific cell types, including cardiac cells and skeletal muscle cells.View product
Biolaminin 521 LN (LN521)
Human recombinant laminin 521
Biolaminin 521 LN is the natural laminin for pluripotent stem cells and therefore reliably facilitates self-renewal of human ES and iPS cells in a chemically defined, feeder-free and animal origin-free stem cell culture system. LN521 is animal origin-free to the primary level.View product

Talk to our team to get a custom proposal
We are here to help you in your journey.